Postmenopausal Vaginal Atrophy Treatment Market | Growth Projections and Innovations 2025 - 2032
Data Bridge Market Research analyses a growth rate in postmenopausal vaginal atrophy treatment market in the forecast period 2023-2030. The expected CAGR of postmenopausal vaginal atrophy treatment market is tend to be around 7.50% in the mentioned forecast period. The market value is USD 2.52 billion in 2022, and it would grow upto USD 4.5 billion by 2030.
Introduction
Postmenopausal vaginal atrophy—commonly described within the clinical construct of genitourinary syndrome of menopause (GSM)—is a chronic condition driven by decreased estrogen and androgen exposure to the vulvovaginal tissues after the menopause transition. Symptoms include vaginal dryness, burning and irritation, dyspareunia (painful intercourse), urinary urgency, and recurrent urinary tract infections; together these effects can severely compromise sexual function, urinary health, and overall quality of life. Prevalence estimates vary by population and definition but large studies report that a substantial fraction of postmenopausal women experience one or more GSM symptoms, and many do not seek care until symptoms become bothersome. 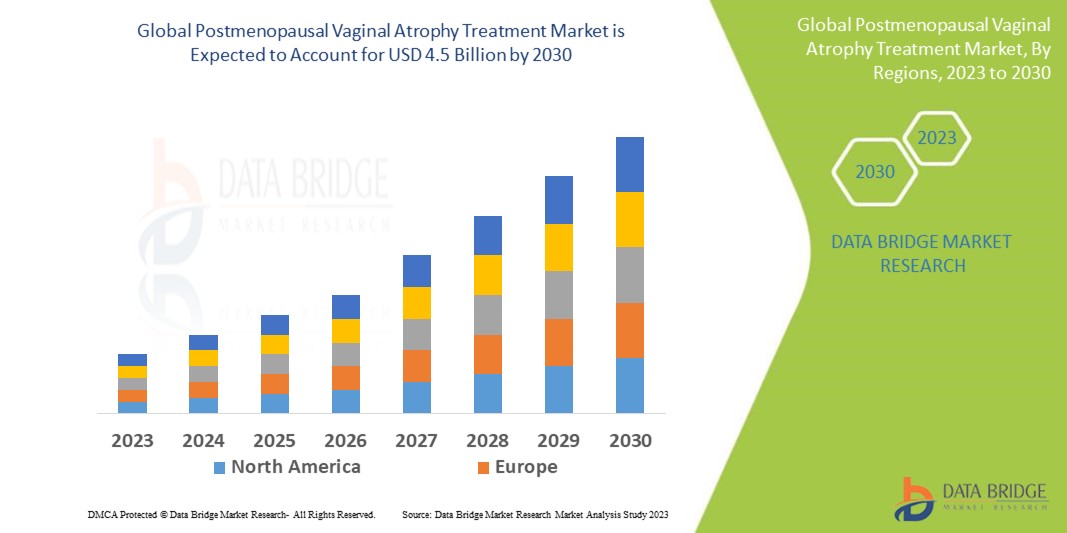
Treatment landscape: established options and newer modalities
Therapies span a spectrum from conservative symptomatic measures to prescription pharmacotherapy and novel procedural approaches:
-
Conservative symptomatic care. Over-the-counter (OTC) vaginal lubricants and long-acting vaginal moisturizers are widely used for immediate relief of dryness and dyspareunia. They are safe, inexpensive, and commonly recommended as first-line measures, though they do not reverse the atrophic changes in vaginal trophism.
-
Local vaginal estrogen. Low-dose vaginal estrogens (creams, tablets, rings) directly restore the epithelium, improve lubrication and elasticity, and are supported by clinical guidelines as a principal therapy for many women with moderate to severe GSM. When used at low local doses, systemic absorption is minimal and local estrogen is effective for most vulvovaginal and some urinary symptoms. Professional guidance emphasizes individualized treatment decisions—particularly in women with a history of hormone-sensitive cancers—and reassures clinicians about the favorable risk/benefit profile of local therapy for typical GSM indications. Non-topical prescription agents. Ospemifene, an oral selective estrogen receptor modulator (SERM), is approved in many countries to treat moderate to severe dyspareunia and vaginal dryness associated with vulvovaginal atrophy, and offers a non-topical, daily oral option for women who prefer systemic administration. Intravaginal prasterone (DHEA) is another prescription local agent that serves as a steroid precursor converted within vaginal tissues and has demonstrated improvement in dyspareunia in randomized trials.
-
Procedural and regenerative approaches. Energy-based devices (fractional CO₂ lasers, erbium:YAG lasers, radiofrequency) and injectables (hyaluronic acid, platelet-rich plasma) are offered in many clinics as non-hormonal, office-based treatments for GSM and for “vaginal rejuvenation.” These therapies have generated commercial momentum and patient interest but remain controversial because randomized, sham-controlled trials and systematic reviews report mixed results and regulators have issued cautions. High-quality evidence and long-term safety data are limited for many procedural approaches, so most professional organizations recommend cautious use within research settings until clearer efficacy and safety profiles are established.
Factors driving demand
Several demographic and clinical trends expand demand for effective GSM therapies:
-
Demographics and prevalence. An increasing global population of postmenopausal women increases absolute need for GSM care; population surveys indicate vaginal dryness and related symptoms affect a large portion of postmenopausal cohorts.
-
Greater help-seeking and decreased stigma. Patient awareness and willingness to discuss sexual health with clinicians have risen in many settings, prompting more women to seek evaluation and treatment rather than silently tolerate symptoms.
-
Clinical contraindications to systemic hormones. The growing number of breast cancer survivors and others for whom systemic estrogen is contraindicated creates demand for validated non-estrogenic or locally acting options and for research into novel non-hormonal therapies.
-
Consumer interest in office procedures and wellness services. The proliferation of aesthetic and wellness clinics offering “vaginal rejuvenation” has introduced a consumer-driven market segment; although uptake may be high in self-pay settings, clinical adoption depends on better evidence and clearer regulatory guidance.
Clinical evidence and guideline positions
The evidence base is strongest for low-dose vaginal estrogen, which is consistently endorsed by recent guideline statements for symptomatic GSM due to its effectiveness and acceptable safety profile for most women. The American Urological Association and supporting panels highlight local estrogen as a principal option, including recommendation in women with certain recurrent urinary problems where local therapy can reduce infections and improve symptoms. For non-hormonal prescription agents, randomized trials support efficacy of intravaginal prasterone and of oral ospemifene for dyspareunia, with known benefit/risk profiles reflected in regulatory labels. Procedural therapies have shown promising but inconsistent outcomes: while some observational and small randomized studies report improvements in symptoms and validated scales, randomized sham-controlled trials and systematic reviews show mixed results, prompting regulatory advisories and professional caution. High-quality, longer-term RCTs remain a priority to clarify relative benefits, durability, and safety of device-based approaches.
Commercial and reimbursement dynamics
The treatment ecosystem comprises prescription drug manufacturers, OTC product makers, device and consumable suppliers, specialty clinics offering procedures, and primary care/gynecologic prescribers. Market access and uptake are shaped by:
-
Reimbursement. Vaginal estrogen products and prescription agents are variably reimbursed across geographies—some are covered on formularies, others require copay or self-pay. Procedural treatments frequently fall into out-of-pocket categories, accelerating consumer pay streams but limiting broad clinical adoption if payers withhold coverage.
-
Regulatory posture. Regulatory warnings and guidance influence clinician and patient uptake—cautions about energy-based devices and the need for clearer labeling shape commercial strategies and clinician education.
-
Evidence for reimbursement negotiation. Pharmaceutical and device companies seeking broader payer coverage emphasize randomized trial evidence, patient-reported outcome benefits, and cost-effectiveness analyses demonstrating improved quality of life and reduced downstream costs (for example, fewer UTIs or reduced need for other symptomatic care).
Patient segmentation and clinical pathways
Clinicians tailor therapy selection based on symptom severity, comorbidities, personal preferences, and contraindications:
-
Mild symptoms: OTC lubricants and moisturizers, education on sexual health, and behavioral measures often suffice.
-
Moderate to severe vaginal atrophy: Local low-dose vaginal estrogen is frequently recommended unless contraindicated; alternatives include intravaginal DHEA or ospemifene for those preferring or needing non-topical options.
-
Contraindications to estrogen: Non-hormonal strategies, participation in clinical trials of regenerative therapies, or specialist multidisciplinary review are typical pathways.
-
Desire for single-visit, non-hormonal options: Some women seek procedural treatments; informed consent, documentation about investigational status, and consideration of cost vs evidence are essential.
Innovation, R&D and digital integration
Active R&D areas and commercial innovations include:
-
Improved local delivery systems (sustained-release rings and low systemic exposure formulations) to enhance convenience and adherence.
-
New tissue-selective compounds and SERMs that retain beneficial vaginal effects with a more favorable systemic safety profile.
-
Regenerative biologics and injectables with ongoing trials assessing efficacy for symptomatic relief and mucosal tissue restoration.
-
Standardized outcome instruments and patient-reported measures that enable head-to-head comparisons and bolster payer discussions.
-
Telemedicine and digital symptom tracking to extend access, improve follow-up, and collect real-world evidence on effectiveness and adherence.
Safety considerations and clinician counseling
Safe, patient-centered counseling is essential. For local estrogen, clinicians typically discuss expected benefits, brief evidence on low systemic exposure at vaginal doses, and individual risk-based decision making—particularly for women with histories of hormone-sensitive malignancy. For procedural therapies, clear, evidence-based counseling about the current state of data, potential risks (burns, scarring, chronic pain) and the investigational nature of many “vaginal rejuvenation” claims is critical. Shared decision-making, documentation of informed consent, and discussion of alternatives (including validated pharmacologic options) protect patients and clinicians.
Market opportunities and constraints
Opportunities exist where evidence, convenience, and reimbursement align: improved local formulations, validated non-hormonal options for women with contraindications, and scalable telemedicine-enabled care pathways can expand responsible access. Constraints include lingering confusion about hormone safety (which affects patient acceptance of local estrogen), variability in payer coverage, regulatory scrutiny of device claims, and the need for higher-quality trials to support long-term safety and durability claims for novel approaches.
Future outlook
Over the near to medium term, expect consolidation around guideline-endorsed pharmacologic therapies while selective adoption of newer modalities advances as evidence accrues. Greater personalization—matching therapy to symptom profile, comorbidity and patient preference—will improve outcomes. Telehealth, validated patient outcomes, and better integration of GSM care into routine menopausal management will reduce under-diagnosis and under-treatment. For device and regenerative fields, randomized sham-controlled studies and long-term safety data will determine which modalities transition from elective, consumer-driven offerings into evidence-based clinical practice.
Conclusion
Postmenopausal vaginal atrophy (GSM) affects many women and carries substantial quality-of-life impact. The therapeutic landscape combines well-established, guideline-backed local estrogen treatments and prescription options (such as ospemifene and intravaginal prasterone) with a rapidly evolving set of non-hormonal and procedural modalities. Clinical uptake, reimbursement, and commercial success depend on robust evidence of efficacy and safety, transparent clinician counseling, and structured pathways that accommodate patient preference and contraindications. As research fills current evidence gaps and digital care pathways expand access, clinicians and companies that prioritize validated outcomes, safety, and patient education will best serve the needs of postmenopausal women worldwide.
FAQs
What are the guideline-recommended first-line therapies for GSM?
How do non-topical options such as ospemifene and prasterone compare with low-dose vaginal estrogen?
What does the current evidence say about the safety and efficacy of vaginal laser treatments?
Which non-hormonal strategies show the most promise for women with contraindications to estrogen?
How should clinicians counsel patients about risks and benefits when considering procedural or regenerative therapies?
Equip yourself with actionable insights and trends from our complete Postmenopausal Vaginal Atrophy Treatment Market analysis. Download now:https://www.databridgemarketresearch.com/reports/global-postmenopausal-vaginal-atrophy-treatment-market
Browse More Reports:
Global Automatic Brewing Equipment Market
Global Automatic Tray Sealing Machines Market
Global Automotive Battery Sensor Market
Global Automotive Cybersecurity Market
Global Automotive Door Impact Bars Market
Global Automotive Drawer Slides Market
Global Automotive Engine Management System Market
Global Automotive Glove Box Market
Global Automotive Intelligence Park Assist System Market
Global Automotive Labels Market
Global Automotive Ozone Generator Market
Global Automotive Rear Cross Traffic Alert System Market
Global Automotive Screen Wash Products Market
Global Automotive Sensors Market
Global Automotive Throttle Cables Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com